Cusabio Human Recombinants
Recombinant Human Nicotinamide mononucleotide adenylyltransferase 3 (NMNAT3) | CSB-EP839418HU
- SKU:
- CSB-EP839418HU
- Availability:
- 13 - 23 Working Days
Description
Recombinant Human Nicotinamide mononucleotide adenylyltransferase 3 (NMNAT3) | CSB-EP839418HU | Cusabio
Alternative Name(s): Nicotinamide-nucleotide adenylyltransferase 3 ;NMN adenylyltransferase 3Nicotinate-nucleotide adenylyltransferase 3 (EC:2.7.7.18) ;NaMN adenylyltransferase 3;Pyridine nucleotide adenylyltransferase 3 (EC:2.7.7.1) ;PNAT-3
Gene Names: NMNAT3
Research Areas: Metabolism
Organism: Homo sapiens (Human)
AA Sequence: MYQVIQGIISPVNDTYGKKDLAASHHRVAMARLALQTSDWIRVDPWESEQAQWMETVKVLRHHHSKLLRSPPQMEGPDHGKALFSTPAAVPELKLLCGADVLKTFQTPNLWKDAHIQEIVEKFGLVCVGRVGHDPKGYIAESPILRMHQHNIHLAKEPVQNEISATYIRRALGQGQSVKYLIPDAVITYIKDHGLYTKGSTWKGKSTQSTEGKTS
Source: E.coli
Tag Info: N-terminal 6xHis-SUMO-tagged
Expression Region: 1-215aa
Sequence Info: Full Length of Isoform 2
MW: 40.1 kDa
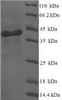
Purity: Greater than 90% as determined by SDS-PAGE.
Relevance: Catalyzes the formation of NAD+ from nicotinamide mononucleotide (NMN) and ATP. Can also use the deamidated form; nicotinic acid mononucleotide (NaMN) as substrate with the same efficiency. Can use triazofurin monophosphate (TrMP) as substrate. Can also use GTP and ITP as nucleotide donors. Also catalyzes the reverse reaction, i.e. the pyrophosphorolytic cleavage of NAD+. For the pyrophosphorolytic activity, can use NAD+, NADH, NaAD, nicotinic acid adenine dinucleotide phosphate (NHD), nicotinamide guanine dinucleotide (NGD) as substrates. Fails to cleave phosphorylated dinucleotides NADP+, NADPH and NaADP+. Protects against axonal degeneration following injury.
Reference: Identification of FKSG76, a novel gene encoding a NMN adenylyltransferase.Wang Y.-G., Gong L.Complete sequencing and characterization of 21,243 full-length human cDNAs.Ota T., Suzuki Y., Nishikawa T., Otsuki T., Sugiyama T., Irie R., Wakamatsu A., Hayashi K., Sato H., Nagai K., Kimura K., Makita H., Sekine M., Obayashi M., Nishi T., Shibahara T., Tanaka T., Ishii S. , Yamamoto J., Saito K., Kawai Y., Isono Y., Nakamura Y., Nagahari K., Murakami K., Yasuda T., Iwayanagi T., Wagatsuma M., Shiratori A., Sudo H., Hosoiri T., Kaku Y., Kodaira H., Kondo H., Sugawara M., Takahashi M., Kanda K., Yokoi T., Furuya T., Kikkawa E., Omura Y., Abe K., Kamihara K., Katsuta N., Sato K., Tanikawa M., Yamazaki M., Ninomiya K., Ishibashi T., Yamashita H., Murakawa K., Fujimori K., Tanai H., Kimata M., Watanabe M., Hiraoka S., Chiba Y., Ishida S., Ono Y., Takiguchi S., Watanabe S., Yosida M., Hotuta T., Kusano J., Kanehori K., Takahashi-Fujii A., Hara H., Tanase T.-O., Nomura Y., Togiya S., Komai F., Hara R., Takeuchi K., Arita M., Imose N., Musashino K., Yuuki H., Oshima A., Sasaki N., Aotsuka S., Yoshikawa Y., Matsunawa H., Ichihara T., Shiohata N., Sano S., Moriya S., Momiyama H., Satoh N., Takami S., Terashima Y., Suzuki O., Nakagawa S., Senoh A., Mizoguchi H., Goto Y., Shimizu F., Wakebe H., Hishigaki H., Watanabe T., Sugiyama A., Takemoto M., Kawakami B., Yamazaki M., Watanabe K., Kumagai A., Itakura S., Fukuzumi Y., Fujimori Y., Komiyama M., Tashiro H., Tanigami A., Fujiwara T., Ono T., Yamada K., Fujii Y., Ozaki K., Hirao M., Ohmori Y., Kawabata A., Hikiji T., Kobatake N., Inagaki H., Ikema Y., Okamoto S., Okitani R., Kawakami T., Noguchi S., Itoh T., Shigeta K., Senba T., Matsumura K., Nakajima Y., Mizuno T., Morinaga M., Sasaki M., Togashi T., Oyama M., Hata H., Watanabe M., Komatsu T., Mizushima-Sugano J., Satoh T., Shirai Y., Takahashi Y., Nakagawa K., Okumura K., Nagase T., Nomura N., Kikuchi H., Masuho Y., Yamashita R., Nakai K., Yada T., Nakamura Y., Ohara O., Isogai T., Sugano S.Nat. Genet. 36:40-45(2004)
Storage: The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself. Generally, the shelf life of liquid form is 6 months at -20?/-80?. The shelf life of lyophilized form is 12 months at -20?/-80?.
Notes: Repeated freezing and thawing is not recommended. Store working aliquots at 4? for up to one week.
Function: Catalyzes the formation of NAD(+) from nicotinamide mononucleotide (NMN) and ATP. Can also use the deamidated form; nicotinic acid mononucleotide (NaMN) as substrate with the same efficiency. Can use triazofurin monophosphate (TrMP) as substrate. Can also use GTP and ITP as nucleotide donors. Also catalyzes the reverse reaction, i.e. the pyrophosphorolytic cleavage of NAD(+). For the pyrophosphorolytic activity, can use NAD(+), NADH, NaAD, nicotinic acid adenine dinucleotide phosphate (NHD), nicotinamide guanine dinucleotide (NGD) as substrates. Fails to cleave phosphorylated dinucleotides NADP(+), NADPH and NaADP(+). Protects against axonal degeneration following injury.
Involvement in disease:
Subcellular Location: Mitochondrion
Protein Families: Eukaryotic NMN adenylyltransferase family
Tissue Specificity: Expressed in lung and spleen with lower levels in placenta and kidney.
Paythway:
Form: Liquid or Lyophilized powder
Buffer: If the delivery form is liquid, the default storage buffer is Tris/PBS-based buffer, 5%-50% glycerol. If the delivery form is lyophilized powder, the buffer before lyophilization is Tris/PBS-based buffer, 6% Trehalose, pH 8.0.
Reconstitution: We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20?/-80?. Our default final concentration of glycerol is 50%. Customers could use it as reference.
Uniprot ID: Q96T66
HGNC Database Link: HGNC
UniGene Database Link: UniGene
KEGG Database Link: KEGG
STRING Database Link: STRING
OMIM Database Link: OMIM