Cusabio Human Recombinants
Recombinant Human Large proline-rich protein BAG6 (BAG6), partial | CSB-BP342402HU
- SKU:
- CSB-BP342402HU
- Availability:
- 3 - 7 Working Days
Description
Recombinant Human Large proline-rich protein BAG6 (BAG6), partial | CSB-BP342402HU | Cusabio
Alternative Name(s): BAG family molecular chaperone regulator 6 (BCL2-associated athanogene 6Imported) (BAG-6) (HLA-B-associated transcript 3) (Protein G3) (Protein Scythe) (BAT3) (G3)
Gene Names: BAG6
Research Areas: Cancer
Organism: Homo sapiens (Human)
AA Sequence: MEPNDSTSTAVEEPDSLEVLVKTLDSQTRTFIVGAQMNVKEFKEHIAASVSIPSEKQRLIYQGRVLQDDKKLQEYNVGGKVIHLVERAPPQTHLPSGASSGTGSASATHGGGSPPGTRGPGASVHDRNANSYVMVGTFNLPSDGSAVDVHINMEQAPIQSEPRVRLVMAQHMIRDIQTLLSRMECRGGPQPQHSQPPPQPPAVTPEPVALSSQTSEPVESEAPPREPMEAEEVEERAPAQNPELTPGPAPAGPTPAPETNAPNHPSPAEYVEVLQELQRLESRLQPFLQRYYEVLGAAATTDYNNNHEGREEDQRLINLVG
Source: Baculovirus
Tag Info: N-terminal 10xHis-tagged and C-terminal Myc-tagged
Expression Region: 1-321aa
Sequence Info: Partial
MW: 38.7 kDa
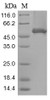
Purity: Greater than 85% as determined by SDS-PAGE.
Relevance: ATP-independent molecular chaperone preventing the aggregation of misfolded and hydrophobic patches-containing proteins. Functions as part of a cytosolic protein quality control complex, the BAG6/BAT3 complex, which maintains these client proteins in a soluble state and participates to their proper delivery to the endoplasmic reticulum or alternatively can promote their sorting to the proteasome where they undergo degradation. The BAG6/BAT3 complex is involved in the post-translational delivery of tail-anchored/type II transmembrane proteins to the endoplasmic reticulum membrane. Recruited to ribosomes, it interacts with the transmembrane region of newly synthesized tail-anchored proteins and together with SGTA and ASNA1 mediates their delivery to the endoplasmic reticulum. Client proteins that cannot be properly delivered to the endoplasmic reticulum are ubiquitinated by RNF126, an E3 ubiquitin-protein ligase associated with BAG6 and are sorted to the proteasome. SGTA which prevents the recruitment of RNF126 to BAG6 may negatively regulate the ubiquitination and the proteasomal degradation of client proteins, the BAG6/BAT3 complex also functions as a sorting platform for proteins of the secretory pathway that are mislocalized to the cytosol either delivering them to the proteasome for degradation or to the endoplasmic reticulum. The BAG6/BAT3 complex also plays a role in the endoplasmic reticulum-associated degradation (ERAD), a quality control mechanism that eliminates unwanted proteins of the endoplasmic reticulum through their retrotranslocation to the cytosol and their targeting to the proteasome. It maintains these retrotranslocated proteins in an unfolded yet soluble state condition in the cytosol to ensure their proper delivery to the proteasome. BAG6 is also required for selective ubiquitin-mediated degradation of defective nascent chain polypeptides by the proteasome. In this context, it may participate to the production of antigenic peptides and play a role in antigen presentation in immune response. BAG6 is also involved in endoplasmic reticulum stress-induced pre-emptive quality control, a mechanism that selectively attenuates the translocation of newly synthesized proteins into the endoplasmic reticulum and reroutes them to the cytosol for proteasomal degradation. BAG6 may ensure the proper degradation of these proteins and thereby protects the endoplasmic reticulum from protein overload upon stress. By inhibiting the polyubiquitination and subsequent proteasomal degradation of HSPA2 it may also play a role in the assembly of the synaptonemal complex during spermatogenesis. Also positively regulates apoptosis by interacting with and stabilizing the proapoptotic factor AIFM1
Reference: "A gene pair from the human major histocompatibility complex encodes large proline-rich proteins with multiple repeated motifs and a single ubiquitin-like domain." Banerji J., Sands J., Strominger J.L., Spies T. Proc. Natl. Acad. Sci. U.S.A. 87:2374-2378(1990)
Storage: The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself. Generally, the shelf life of liquid form is 6 months at -20?/-80?. The shelf life of lyophilized form is 12 months at -20?/-80?.
Notes: Repeated freezing and thawing is not recommended. Store working aliquots at 4? for up to one week.
Function: ATP-independent molecular chaperone preventing the aggregation of misfolded and hydrophobic patches-containing proteins
Involvement in disease:
Subcellular Location: Cytoplasm, cytosol, Nucleus, Secreted, exosome
Protein Families:
Tissue Specificity: Expressed by immature dendritic cells (at protein level).
Paythway:
Form: Liquid or Lyophilized powder
Buffer: If the delivery form is liquid, the default storage buffer is Tris/PBS-based buffer, 5%-50% glycerol. If the delivery form is lyophilized powder, the buffer before lyophilization is Tris/PBS-based buffer, 6% Trehalose, pH 8.0.
Reconstitution: We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20?/-80?. Our default final concentration of glycerol is 50%. Customers could use it as reference.
Uniprot ID: P46379
HGNC Database Link: HGNC
UniGene Database Link: UniGene
KEGG Database Link: KEGG
STRING Database Link: STRING
OMIM Database Link: OMIM