Cusabio Human Recombinants
Recombinant Human Heme oxygenase 1 (HMOX1), partial | CSB-EP010583HU
- SKU:
- CSB-EP010583HU
- Availability:
- 3 - 7 Working Days
Description
Recombinant Human Heme oxygenase 1 (HMOX1), partial | CSB-EP010583HU | Cusabio
Alternative Name(s): 32 kD; bK286B10; D8Wsu38e; heat shock protein 32 kD; heat shock protein 32kD; Heat shock protein; Heme oxygenase (decycling) 1; Heme oxygenase 1; Hemox; HMOX 1; Hmox; Hmox1; HMOX1_HUMAN; HO 1; HO; HO-1; HO1 ; Hsp32
Gene Names: HMOX1
Research Areas: Cardiovascular
Organism: Homo sapiens (Human)
AA Sequence: RPQPDSMPQDLSEALKEATKEVHTQAENAEFMRNFQKGQVTRDGFKLVMASLYHIYVALEEEIERNKESPVFAPVYFPEELHRKAALEQDLAFWYGPRWQEVIPYTPAMQRYVKRLHEVGRTEPELLVAHAYTRYLGDLSGGQVLKKIAQKALDLPSSGEGLAFFTFPNIASATKFKQLYRSRMNSLEMTPAVRQRVIEEAKTAFLLNIQLFEELQELLTHDTKDQSPSRAPGLRQRASNKVQDSAPVETPRGKPPLNTRSQAPLLRWVLTLSFLVATVAVGLYAM
Source: E.coli
Tag Info: N-terminal 6xHis-tagged
Expression Region: 3-288aa
Sequence Info: Partial
MW: 36.6 kDa
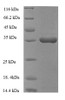
Purity: Greater than 90% as determined by SDS-PAGE.
Relevance: He oxygenase cleaves the he ring at the alpha methene bridge to form biliverdin. Biliverdin is subsequently converted to bilirubin by biliverdin reductase. Under physiological conditions, the activity of he oxygenase is highest in the spleen, where senescent erythrocytes are sequestrated and destroyed. Exhibits cytoprotective effects since excess of free he sensitizes cells to undergo apoptosis.
Reference: Initial characterization of the human central proteome.Burkard T.R., Planyavsky M., Kaupe I., Breitwieser F.P., Buerckstuemmer T., Bennett K.L., Superti-Furga G., Colinge J.BMC Syst. Biol. 5:17-17(2011)
Storage: The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself. Generally, the shelf life of liquid form is 6 months at -20?/-80?. The shelf life of lyophilized form is 12 months at -20?/-80?.
Notes: Repeated freezing and thawing is not recommended. Store working aliquots at 4? for up to one week.
Function: Heme oxygenase cleaves the heme ring at the alpha methene bridge to form biliverdin. Biliverdin is subsequently converted to bilirubin by biliverdin reductase. Under physiological conditions, the activity of heme oxygenase is highest in the spleen, where senescent erythrocytes are sequestrated and destroyed. Exhibits cytoprotective effects since excess of free heme sensitizes cells to undergo apoptosis.
Involvement in disease: Heme oxygenase 1 deficiency (HMOX1D)
Subcellular Location: Microsome, Endoplasmic reticulum membrane, Peripheral membrane protein, Cytoplasmic side
Protein Families: Heme oxygenase family
Tissue Specificity: Expressed at higher levels in renal cancer tissue than in normal tissue (at protein level).
Paythway: HIF-1signalingpathway
Form: Liquid or Lyophilized powder
Buffer: If the delivery form is liquid, the default storage buffer is Tris/PBS-based buffer, 5%-50% glycerol. If the delivery form is lyophilized powder, the buffer before lyophilization is Tris/PBS-based buffer, 6% Trehalose, pH 8.0.
Reconstitution: We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20?/-80?. Our default final concentration of glycerol is 50%. Customers could use it as reference.
Uniprot ID: P09601
HGNC Database Link: HGNC
UniGene Database Link: UniGene
KEGG Database Link: KEGG
STRING Database Link: STRING
OMIM Database Link: OMIM